Learning Center
Colloidal Silver - Ions vs. Particles
 Colloidal
silver contains silver in two distinctly different forms:
Colloidal
silver contains silver in two distinctly different forms:
- silver nanoparticles
- silver ions
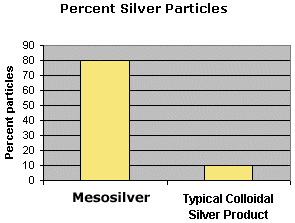
The silver content of Mesosilver is typically 80% nanoparticles and 20% silver ions. The total silver concentration is 20 ppm. This amount of silver in the nanoparticles is uncommonly high compared to other commercially available colloidal silver products. CSL has measured the ionic content in hundreds of samples of colloidal silver. Virtually all other products fall in the range between 75 to 99 percent ionic, the most typical value being 90 percent ionic.
Ionic silver is not metallic silver as found in the particles, but is dissolved silver. A silver ion is a single atom of silver that is missing one orbital electron. The number and arrangement of orbital electrons of a silver atom determines its physical properties. When an electron is removed the physical properties of the silver is altered dramatically.
For example, metallic silver will not dissolve in water, but ionic silver can only exist when it is dissolved in water. A major difference between ions and particles is what happens when the water containing them is evaporated.
What happens to the silver ions in solution when the water is evaporated?
Silver ions in a solution cannot exist without water, so when the water is evaporated the silver ions (cations) must combine with an available anion to form a compound. The predominant anions present in a silver colloid solution are hydroxide and carbonate. The compounds thus formed are silver hydroxide and silver carbonate. Silver hydroxide is unstable and reduces to silver oxide and hydrogen. The silver carbonate will reduce to silver oxide and carbon dioxide. The final compound that remains is silver oxide.
This process begins as a single silver ion is forced to combine with a single anion forming a single molecule of the compound. The molecule has no ionic charge and therefore no repulsive force. The lack of repulsion causes the molecules to be attracted to each other by van der Waals' force of attraction which causes them to aggregate and form small particles of the compound. The size of the particle growth is limited by the reduced mobility of the molecules as the water evaporates. What remains is particles of silver oxide whose diameter is 1 - 3 nanometers. When ionic silver solutions are used topically, the water will evaporate within a few minutes leaving a film of silver oxide. Silver oxide possess minimal anti-bacterial properties so the effectiveness of the ionic silver is essentially lost.
What happens to the silver particles in Mesosilver when the water is evaporated?
When the water is evaporated from Mesosilver, the nanometer sized silver particles form a thin film of pure metallic silver on the surface that was wetted with before evaporation took place. Metallic silver particles in the form of a thin film retain the anti-bacterial properties of silver and thus protect the surface from bacterial growth. This is very important for disinfectant applications where the anti-bacterial properties of the thin silver film that remains on the surface after the water is evaporated continues to provide protection against bacterial growth. By contrast, the silver oxide that remains when ionic silver is evaporated offers very little protection.
For a detailed list of differences between silver particles and silver ions, see the Summary of Properties table.




