Learning Center
Structure of the Silver Atom
Chemical Symbol: Ag
Atomic Number: 47
Atomic Weight/Mass: 107.8682 amu
Standard State: Solid, at 298 Degrees Kelvin ( 77 degrees Fahrenheit )
CAS ID: 7440-22-4
Group Number: 11
Class: Metal, Transition
Melting Point of Silver: 961.93 °C
Boiling Point of Silver: 2,212.0 °C
Number of Protons & Electrons: 47
Number of Nuetrons: 61
Crystal Structure of Silver: Cubic ( face-centered )
Density @ 293 K: 10.5 g/cm3
Atomization: 284.09 kJ mol-1
Fusion: 11.3 kJ mol-1
Vaporization: 257.7 kJ mol-1
Molar volume: 10.335 cm3 mol-1
Electrical resistivity: 1.59 µ-ohms/cm
Thermal conductivity: 429.0 W / m / K
Mass magnetic susceptibility: -2.27e-9
Number of Energy Levels/Valence Shells: 5
Ground state electron configuration: 4d10 5s1
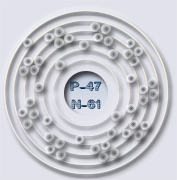
The silver atom has 5 electron orbits (energy levels) with a total of 47 electrons. Beginning with the orbit closest to the nucleus and working outward, the number of electrons per orbit should be: 2, 8, 18, 18, 1. Of course, the nucleus contains 47 protons and 61 neutrons.
Silver Nuclear Magnetic Resonance ( Nuclide 107 ):
Frequency: 4.046 MHz ( Ag+ )
Silver Incompatiblities:
Acetylene, ammonia, hydrogen peroxide, bromoazide, chlorine trifluoride, ethyleneimine, oxalic acid, tartaric acid




